PAT Technology FAQ
How does WatchPAT detect Apnea, Hypopnea, and Respiratory Effort Related Arousal (RERA) events?
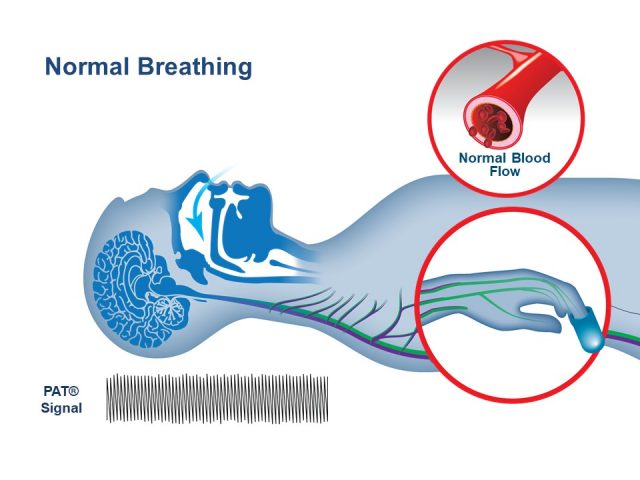
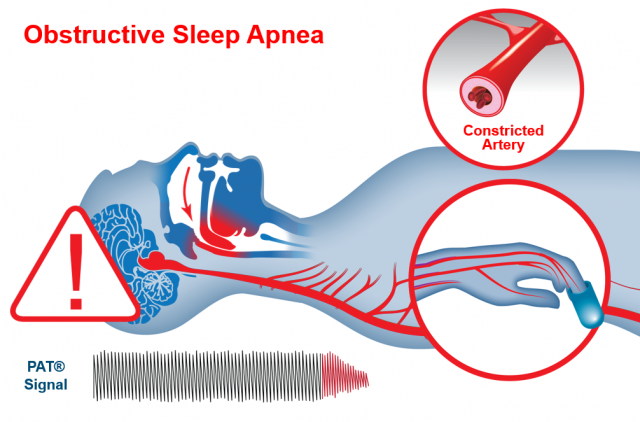
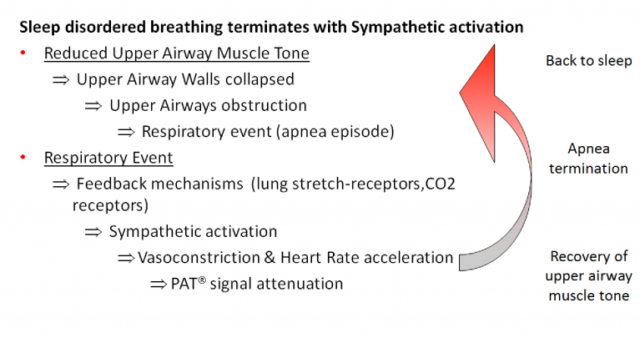
WatchPATM utilizes Peripheral Arterial Tone (PAT), a special physiological signal that mirrors changes in the autonomic nervous system (ANS) caused by respiratory disturbances during sleep. The ANS regulates many of our basic functions and it does this without our conscious control. Among its activities are the regulation of blood vessel size and blood pressure, airflow in the lungs, and the heart’s electrical activity and ability to contract. WatchPAT’s automatic algorithm analyzes the PAT signal amplitude along with the heart rate and oxygen saturation to identify and classify breathing problems while you sleep. Using specific signal patterns, the algorithm provides two indices that allow a diagnosis of sleep apnea: • AHI (Apnea/Hypopnea Index), which is an index used to calculate sleep apnea severity based on the total number of complete cessations (apneas) and partial obstructions (hypopneas) of breathing per hour of sleep. • RDI (Respiratory Disturbance Index) is used to assess the severity of sleep apnea by measuring respiratory efforts, or RERAs (Respiratory Effort Related Arousals). A RERA is an arousal from sleep that follows 10 seconds or more of increased respiratory effort but does not meet the criteria for apnea or hypopnea. The snoring sensor enables the clinician to determine if the respiratory events are obstructive and the body position sensor enables the clinician to determine if there is a positional component to the sleep apnea
How does WatchPAT detect rapid eye movement (REM) from the finger?
Rapid eye movement (REM) sleep is associated with considerable attenuation of the PAT signal and physiology coupled with specific variations in the PAT amplitude and rate. Based on this specific variability in the PAT and pulse rate signals, REM sleep stage is differentiated from non-REM sleep. In addition, it is differentiated from the wake state by WatchPAT’s advanced actigraphy algorithms. This is clinically important to prevent under-treatment of apnea if predominantly REM-related.
How does the WatchPAT home sleep apnea test detect sleep architecture?
WatchPAT’s sleep/wake detection is based on data recorded by the built-in actigraph. The propriety software’s automatic actigraph algorithm discriminates between sleep and wake states in normal subjects and those with Obstructive Sleep Apnea (OSA). This algorithm makes WatchPAT’s home sleep apnea test (HSAT) superior to all other actigraph devices—most of them fail when used with OSA subjects. WatchPAT’s sleep/wake algorithm has been validated and published in peer-reviewed journals. The results show very good agreement between actigraphy and PSG in determining sleep efficiency, total sleep time, and sleep latency (agreement 86% in normal subjects, 86%-mild OSA, 84%-moderate OSA, 80% severe OSA).
How does WatchPAT differentiate between respiratory arousals and other events like Periodic Limb Movement (PLM) related arousals and spontaneous arousals?
The algorithms differentiate between respiratory related arousals and Periodic Limb Movement (PLM) related or spontaneous arousals via detection of specific patterns in the PAT signal coupled with the presence or absence of unique dynamics of the pulse oximetry signal.