By Rhonda Welch, CPC

Home Sleep Apnea Tests (HSAT) have increased in utilization and based on recent estimates amount to about a third of all sleep apnea tests due to their cost effectiveness and accessibility in comparison to in lab, attend Polysomnography testing. An HSAT is a preference for many patients since they can take the test at home in a more natural, relaxing and private environment that is also more likely to reflect the actual disease manifestation.
Today, the vast majority payers reimburse for HSAT and some recommend it as first line diagnosis for sleep apnea. However their coding and billing requirements differ from payer to payer. It is always best to check with your payer for their specific requirements, but this article will outline some of the basics.
With few exceptions, licensed medical doctors, regardless of the their specialty, can prescribe HSAT to patients who are suspected of sleep apnea based on sign and symptoms and testing positive for high risk on validated instruments such as the STOPBANG, Epworth Sleepiness and DOISNORE 50 questionnaires. In addition, physicians may also consider clinical symptoms such as atrial fibrillation and hypertension as signs for high pre-test probability, based on the most recent AASM guidelines1.
HSAT G Codes and CPT Codes
In 2007 the American Academy of Sleep Medicine (AASM) published the “Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients”2 which differentiated the HSATs by type (defined by the AASM). In the following year, Medicare introduced the HCPCS Level II codes G0398, G0399 and G0400 which followed the AASM types. G codes are “carrier determined” which means that payment is up to the discretion of the Medicare Administrative Contractors (MACs).
G codes / Sleep Type Classification
G0398 Home sleep study with type II portable monitor, unattended; minimum of 7 channels: EEG, EOG, EMG, ECG/heart rate, airflow, respiratory effort and oxygen saturation.
G0399 Home sleep study with type III portable monitor, unattended; minimum of 4 channels: 2 respiratory movement/airflow, 1 ECG/heart rate and 1 oxygen saturation.
In 2009, CMS issued a National Coverage Determination (NCD) which called out the WatchPAT as a covered test.
Today, most CMS MACs request the use of G codes to report HSATs and request the use of G0400
to report WatchPAT.
In 2011, the AMA added the CPT codes 95800 and 95801 to describe HSAT using peripheral arterial tone (ie WatchPAT). Note that WatchPAT records sleep time so CPT 95800, not 95801 should be used to report HSAT using WatchPAT. Most commercial payers request the use of 95800 to report WatchPAT.
CPT Codes
95800 Sleep study, unattended, simultaneous recording; heart rate, oxygen saturation, respiratory analysis (eg. by airflow or peripheral arterial tone), and sleep time.
95801 Sleep study, unattended, simultaneous recording; heart rate, oxygen saturation, respiratory analysis (eg. by airflow or peripheral arterial tone).
In 2017 AASM clarified their position in a guideline, noting that the types classification fails to consider new technologies such as peripheral arterial tonometry (PAT). They proposed another classification scheme but acknowledged that it has not been utilized by many. The AASM concluded that devices that measure PAT, actigraphy, and oximetry are technically adequate to diagnose OSA and therefore recommended that physicians use such HSATs to diagnose OSA.3
The bottom line is that there are multiple codes that can be used to report HSAT. Typically, CMS requests that WatchPAT be reported with G0400 and commercial payers request 95800. Since HSAT may reported by more than one code, it is best to refer to the payer’s medical policy to ensure you are reporting the correct code.
In upcoming newsletter editions, we will explore additional topics including when to bill the global, technical and professional components of the code, credentialing and accreditation issues.
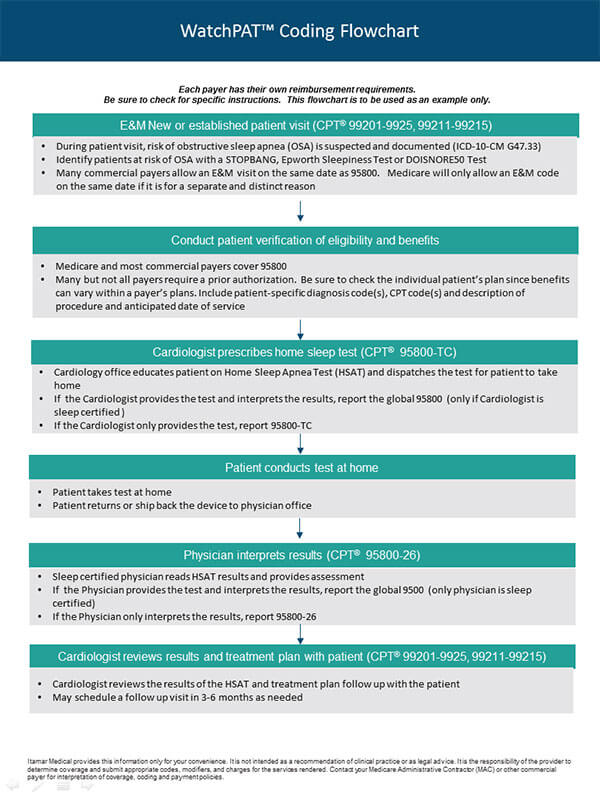
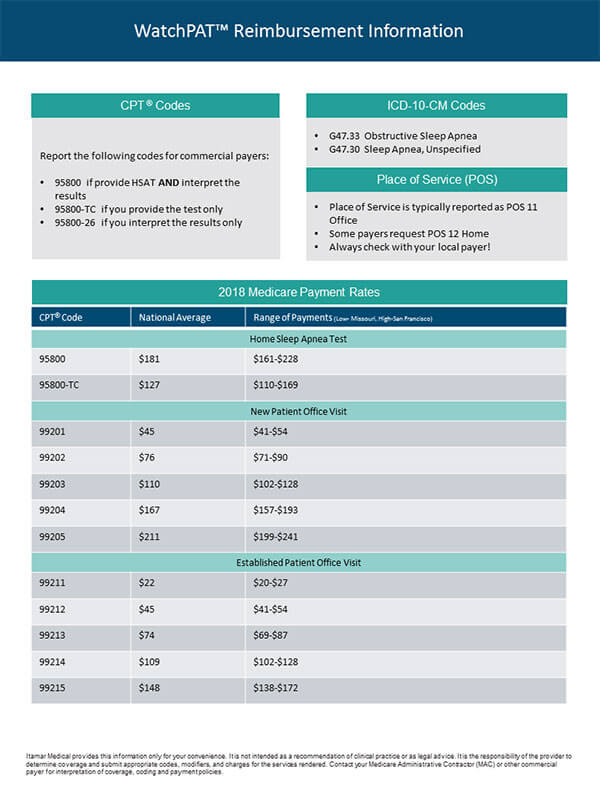
[1] Kapur et al., Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline, 13 J. Clin. Sleep Med. 479 (Mar. 15, 2017).
[2] Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients. JCSM Journal of Clinical Sleep Medicine, Vol. 3, No. 7, 2007.
[3]CMS Pub 100-03 Medicare National Coverage Determinations (NCD) – Sleep Testing for Obstructive Sleep Apnea (OSA). Transmittal R103NCD March 3, 2009
