
Poor quality sleep can lead to diabetes
Genetics, weight gain, and diet are often associated with the risk for diabetes. But, according to new studies, the lack of sleep can also increase
Home » Healthcare Professionals » Sleep Apnea Blog
Read our Sleep Apnea Blog to learn more about home sleep apnea testing (HSAT), industry news, and clinical research about obstructive and central sleep apnea.

Genetics, weight gain, and diet are often associated with the risk for diabetes. But, according to new studies, the lack of sleep can also increase

The answer may be “yes.” A recent study found that older adults who received positive airway pressure (PAP) therapy prescribed for obstructive sleep apnea (OSA)

Men and women reacting differently to the same situation isn’t news. But men and women reacting differently to the same medical condition? A recent article
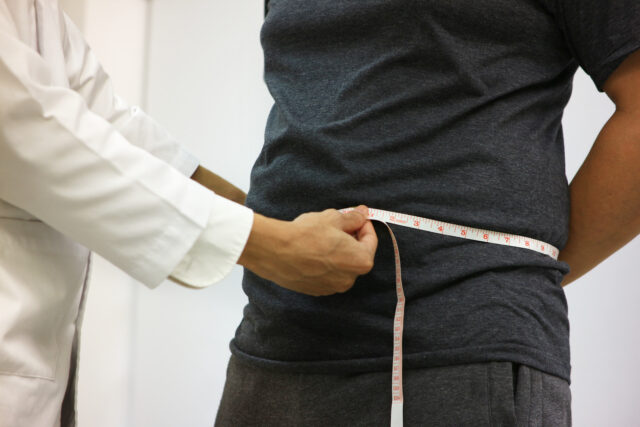
Obstructive sleep apnea (OSA) is well-established as a disease that is also a risk factor for many other health problems. Studies continue to help increase
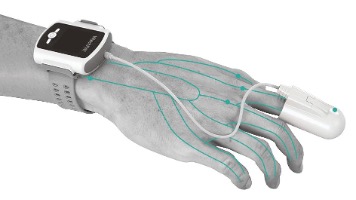
Itamar Medical’s WatchPAT® home sleep apnea test (HSAT) devices continue to grow in popularity among prescribers and patients, for many reasons. Patients can appreciate the

A landmark study presented at the European Respiratory Society (ERS) International Congress 2021 says “using PAP (positive airway pressure) therapy as directed can significantly increase
Many Americans have trouble falling asleep or staying asleep.1 The reasons for that are many and varied—stress, illness, pain, some medications, hot flashes or hormone

Over one billion individuals worldwide experience some form of sleep apnea, and the number is rising. Obstructive sleep apnea (OSA) can negatively influence quality of

Higher physical activity and less time spent sitting and watching TV was associated with a significantly lower risk of developing obstructive sleep apnea.1 But is

Association between sleep apnea and blood pressure control in African Americans African Americans have a high prevalence of both hypertension and uncontrolled blood pressure (BP),

Study shows Hispanics/Latinos at greater risk of long-term cognitive decline from poor sleep Poor sleep impacts the risk of long-term cognitive decline in Hispanic/Latino middle-aged

The American Academy of Sleep Medicine (AASM) has released a bold new position statement declaring that sleep is a biological necessity, and insufficient sleep and
